Part 2 in our blog series on Food Traceability is from Tejas Bhatt, one of our guest speakers at our Food Traceability Summit that is taking place right here in Brighton, Michigan on August 19th. The original source is from “A Guidance Document on The Best Practices in Food Traceability, Comprehensive Reviews in Food Science and Food Safety, Vol. 13, pp. 1074-1103 by Jianrong Zhang and Tejas Bhatt”. We are breaking down the document into different blogs leading up to the event. This section talks about other food regulations in different parts of the world. If you missed part one, you can find the link here.
Global regulations and industry implementation
Regulatory landscape. The requirements of the traceability regulations vary worldwide.
When it comes to recordkeeping, the countries of the European Union (EU) have established the most comprehensive systems, while other nations maintain varying—generally less extensive—requirements. Beyond food safety, recordkeeping also supports broader objectives such as sustainability and consumer information, including sustainability labeling. In Canada, traceability regulations currently apply only to livestock, with no explicit traceability rules in place for other food products. Instead, the traceability of processed foods is maintained through proper packaging and labeling under the Consumer Packaging and Labelling Act, as well as through the Food Safety Enhancement Programs (FSEP) for meat products. Increasingly, businesses are also adopting digital tools, such as mobile device management solutions, to streamline compliance, enhance recordkeeping accuracy, and ensure that regulatory information is securely accessible across the supply chain.
The government of China is gradually establishing and improving national food safety laws and regulations with new requirements on food traceability. In 2009, the “Food Safety Law of PRC” and its implementing regulations was promulgated, requiring food producers to establish a purchase-inspection record system and a food-delivery inspection record system, and accurately record matters of law, or retain relevant notes on every purchase or sale (Liu and others 2012). Other regulations call for traceability requirements mainly at the trading event; there are no specific requirements for internal data collection. Also, there are no requirements for the collection of specific data relating to processing.
In the EU, driven partly by increasing consumer demands, there are many legislative requirements regarding traceability. There is variation among the rules regarding different aspects of trace-ability; most, however, require a one-step-forward–one-step-back approach as well as lot-based traceability. Motivation and scope of these rules cover sustainability and safety aspects as well as food and nonfood products. The general principles for food traceability, established in Article 18 of EC Regulation Number 178/2002, require that the traceability of food be established at all stages of production, processing, and distribution.
Beef and rice products are the only food products addressed in traceability requirements in Japan. The Japanese Ministry of Agriculture, Forestry and Fisheries (MAFF) mandates, under its Beef Traceability Program for Domestic Beef, that an assigned number is carried through from livestock birth to the carcass at the abattoir, and the label or invoice with the final packaged product. With the assigned identification number, a consumer can access the information online and review the history of purchased beef products. The Rice Law of 2009 requires record keeping of transactions (purchases and sales) of rice and grains and informing consumers and business partners of origin information, to allow prompt identification of distribution route when needed. Other than the beef and rice products, there are no regulations for other food products on the traceability requirement.
In the United States, the Bioterrorism Act of 2002 (BT Act), and the recordkeeping requirements contained in the Act, rep-resented a major step forward in the implementation of a product tracing system for FDA-regulated food products. People who “manufacture, process, pack, transport, distribute, receive, hold, or import food” in the United States, as well as foreign food transporters in the United States, are required to maintain records to identify previous sources and subsequent recipients of the food. Required records for food received and released must include: previous/subsequent source (including full contact details), description of food (brand name and variety), date received/released, lot or code number (if relevant), quantity and packaging. When food is released, records additionally must include information “reasonably available” identifying specific sources of each ingredient for each lot of finished product. In cases where food processors commingle ingredients such as flour from different suppliers, FDA accepts that manufacturers may not be able to identify one specific source.
Industry implementation
Other than EU countries which have clear food traceability requirements for recordkeeping in the regulations, various types of public–private partnerships exist to advance food traceability in different food sectors in different countries, such as the U.S. Dairy Traceability and the Meat and Poultry Data Standards Organization.
The MAFF published in 2007 the Japanese Handbook for Introduction of Food Traceability Systems, which provides comprehensive guidance to food standards in accordance with Japanese Agricultural Standards (JAS) and traceability requirements. This reference document outlines the types of information (such as product names, dates, and supplier/customer identifiers) needed to ensure traceability. The handbook also highlights the importance of connecting inputs to outputs to ensure that the critical links in traceability are not broken.
In the United States, several industry initiatives have evolved, and guidance documents were developed. The Produce Traceability Initative (PTI) is carried out by volunteer-led working groups in the areas of Implementation, Master Data, Technology, and Communications. The initiative is administered by the Canadian Produce Marketing Assn. (CPMA), GS1 US, Produce Marketing Assn. (PMA), and United Fresh Produce Assn. (UFPA). In 2010, GS1 published the “Traceability for Fresh Fruits and Vegetables Implementatin Guide” (GS1 2010). Several sector-specific guidances, produced by other organizations, are also available. These are: Guidance for Dairy Product Enhanced Traceability, Trace-ability for Dairy, Deli, and Bakery U.S. Implementation Guide, Traceability for Meat and Poultry: U.S. Implementation Guide; and U.S. Seafood Implementation Guide.
Each of these guidance documents is based on the use of GS1 global standards for supply-chain management and product identification. A guidance particularly focused on CTEs was published in 2010 as “mpXML Traceability Guide (mpXML 2010).” This mpXML guide provides detailed examples of information captured for events common to each supply-chain segment, and illustrates how CTE methodology could be adapted to fit industry practices, such as in-store product transformation and direct-store delivery of products.
Based on a review of government regulations, it appears that a one-step-up and one-step-down approach is a minimum traceability requirement. No current regulations have a specific data requirement along the entire supply chain. Furthermore, there are no uniform requirements for CTE/KDE information collection along the supply chain, either across different sectors or between countries. Other countries, such as China and Japan, have guidance/standards that focus on specific data collection and they address food safety, food sustainability, and customer information.
The U.S. industry initiative and guidance focus on implementation guidance using the GS1 system, which requires an electronic system and considerable investment for small businesses and which is focused mainly on food safety. There is no single document that addresses different food sectors in such a way as to identify the uniform data requirements for food traceability to allow all the stakeholders in the supply chain (farmers, processors, retailers, food service operators, distributors) to have guidance in identifying the CTE/KDE in their operations. This report identifies the CTE/KDE in 6 different food sectors and from the perspectives of different countries, shows the similarities and differences among them, and provides government agencies around the world and the industry with a uniform CTE/KDE data-collection framework.
Concept
As the concept is to cover several different food sectors’ industry practices for traceability, we established a team comprised of experts from different stakeholders to provide the information, guidance, and recommendations.
Assemble project work groups:
Six project work groups consisting of subject matter experts (SME) were assembled to provide sector-specific direction and content for the guidance document. A 7th work group was established to serve as an overview review.
The 6 sector-specific project work groups represented the following food sectors: bakery, dairy, meat/poultry, processed food, produce, and seafood. An additional project work group served as overview reviewers.
The overview review work group was comprised of individuals having expertise in related sectors—distribution, transportation, retail, standards, technology, and regulatory—and able to provide other perspectives (for example, those of small-, mid-, and large-sized companies, international organizations, and farm in-put and ingredient suppliers). Other key stakeholders— Global Food Traceability Center (GFTC) Advisory Council members, and consumer advocacy group representatives—were also included in the overview work group.
Develop a general traceability guidance framework:
KDEs and CTEs, defined by IFT in a pilot study for the U.S. FDA, were used as general baseline and were provided to the sector-specific working groups.
Develop a sector-specific traceability guidance framework:
Project working groups applied the generic traceability framework to the respective food sectors. The SMEs provided content for sector-specific guidance and content for validation, verification, and refinement of the generic traceability framework.
Update the generic traceability guidance framework:
Update the original generic framework using the similarities and differences observed within the specific traceability guidance frameworks for each sector to enhance its applicability and usability.
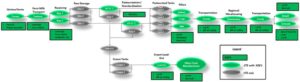
Baseline CTE/KDE framework
There are various points in a supply chain at which data capture is necessary to follow product movement. These points, referred to as CTEs, include:
Shipping from one facility and receiving at another facility (Transportation);
Changes that occur as products are manufactured or transformed during processing (Transformation); and
Trace forward requires an accounting of all products; therefore, it is important for firms to also record the ways in which products exit the supply chain through depletion events.
At each CTE, KDEs must be captured to enable tracking and tracing of product movement through the supply chain.
The concepts of CTEs and KDEs were proposed by IFT in 2010, and considerable effort has been expended over the years by at least 100 stakeholders to clearly identify which data elements need to be provided to regulators in order to effectively trace food products throughout the supply chain (McEntire 2010).
This KDE/CTE framework provides information on the what, where, and when with respect to food products that traverse the supply chain. While each firm must maintain these records inter-nally, these data also establish the links needed to connect supply-chain partners. Cross-sector collaboration must be encouraged to assist industry in sharing best practices and identifying a consistent implementation approach to product tracing for growers, producers, processors, distributors, retailers, and food service operators.
The CTEs and KDEs, along with the guidance to facilitate understanding and implementation, will allow individual supply-chain companies to correctly identify the CTEs that they are responsible for and ensure that KDEs for each CTE are captured and available for reporting as needed based on a specific request from regulatory officials. For definitions of CTEs and KDEs, refer to the IFT FDA pilot report (Bhatt and others 2013).
The following concepts are useful in defining CTEs and suggest that the identified KDEs be considered a minimum standard.
Transportation events. Those events that typically support external product tracing between supply-chain locations resulting from the physical transport of product by air, truck, rail, or ship from one supply-chain location to another supply-chain location.
Shipping CTE: The event at which traceable product is dis-patched from one defined location to another defined location. Shipping CTEs are typically followed by subsequent receiving events. Typically, this event occurs when a traceable product is transported by air, truck, rail, or ship from one supply-chain company to another supply-chain company, al-though it can also be between 2 separate locations within the same company.
Receiving CTE: The event at which traceable product is received at a defined location from another defined location. Receiving CTEs typically occur in response to earlier shipping events. Typically, this event occurs when a traceable product is received at a location after being transported by air, truck, rail, or ship from one supply-chain company to another supply-chain company, although it can also be between 2 separate locations within the same company.
Transformation events. Transformation events support internal product tracing within the 4 walls of a company connecting incoming to outgoing shipments. Examples of transformation events are when product ingredients from one or more suppliers or sources are combined, or when a product is further processed such as by cutting, cooking, or repackaging.
Transformation Input (T1) CTE: The event at which inputs (ingredients) from one or more suppliers or sources are combined and/or processed to produce a new traceable product that enters the supply chain. The objective is to capture the supplier, product identification (ID), and production unit of all ingredients used to create the new traceable product.
Transformation Output (T2) CTE: The event at which outputs (finished product) are created and packaged for entry into the supply chain. The objective is to capture the Supplier, Product ID, and Lot/Batch Number (or equivalent) of the new output product and to ensure this information is available for capture in subsequent T1 events, Transport events, and Depletion events.
Every T1 CTE has a corresponding T2 CTE (that is, every input connected to its corresponding output). Transformation information may be consolidated to levels that the manufacturer feels are adequate to fully link traceable product being used during the Transformation process for the new traceable product being produced. Traceable product produced as an internal use only item during the Transformation process but then used during a subsequent step may not need to be recorded if adequate records are maintained that link the initial traceable product used and the final traceable product created.
Depletion events. Those events that capture how traceable product is removed from the supply chain. Depletion events consist of the following:
Consumption CTE: Those events at which a traceable product becomes available to consumers. Examples of a consumption event include: when a case of fresh produce is opened and placed in bulk self-service bins at a retail grocery store, a packaged traceable product is sold at a point-of-sale (POS) register at a retail grocery store, or a case of seafood product is opened for use in preparing menu items in a restaurant. The objective is to capture the Supplier, Product ID, and Batch/Lot Number (or equivalent) of the traceable product and associate those with the Location, Date, and Time that the product became available to consumers.
Disposal CTE: Those events at which a traceable product is destroyed or discarded or otherwise handled in a manner that the product can no longer be used as a food ingredient or become available to consumers. An example of a Disposal event is when a case of unopened fresh produce or other traceable product at a restaurant or retail store reaches its expiration date and is properly discarded. The objective is to capture the Supplier, Product ID, and if possible, the Batch/Lot Number (or equivalent) of the traceable product and associate those with the Location, Date, and Time that the product was removed from the supply chain without becoming available to consumers. While not used in a trace-back investigation, the Disposal CTE is important during a trace-forward/recall investigation to prove that 100% of items are accounted for.
Also read – How Food Traceability Solutions Help Maintain Customer Confidence


